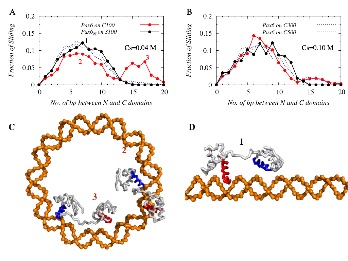
The most intriguing aspect of protein-DNA interactions is that DNA binding proteins (DBPs) can rapidly search and specifically recognize target DNA sites (their cognate sites) among an astronomically large number of nonspecific DNA sequences. The association rate is orders of magnitude faster than estimated for pure three-dimensional diffusion-limited reactions. This led to the idea of facilitated diffusion, during which DBPs lower the dimension of their search space by performing 1D-diffusion along the DNA contour (“sliding”) interspersed by 3Ddissociation. The 3D events can further be considered as a combination of dissociation-reassociation events of short life spans (“hopping”), inter-segmental “jumps” between nearby DNA segments, and larger volume 3D-diffusions. Despite the considerable advancements made in understanding the molecular interactions that underpin protein search dynamics on DNA molecules, the impact of DNA conformation on the same is less explored. Our goal is to investigate the role of DNA conformations, its shape and dynamics on the protein-DNA recognition process through the development of computational models at various levels of complexity.