 |
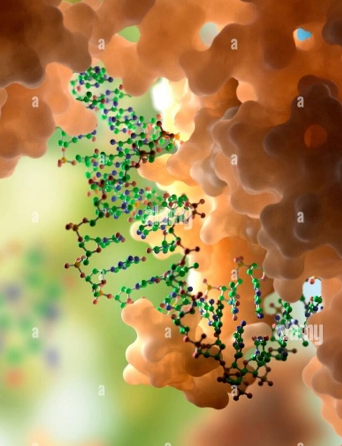
Protein-Nucleic Acid InteractionsIt's fascinating to realize how the incredibly intricate physics of living systems is encoded by a simple four-letter alphabet of nitrogenous bases: Adenine (A), Guanine (G), Cytosine (C), and Thymine (T) in deoxyribonucleic acid (DNA). The specific sequence of these bases carries the essential information for life, which is precisely decoded during critical DNA metabolic processes. The fundamental step in this decoding involves distinguishing meaningful sequences of bases from the vast array of non-essential ones, a task performed by a specialized class of proteins known as DNA-binding proteins (DBPs). While this might appear to be straightforward textbook knowledge, in reality, it represents a complex cellular phenomenon that requires both specificity and timely responses to initiate vital processes such as transcription, replication, and DNA damage repair. Through a combination of in silico models, advanced molecular simulation techniques, and principles from statistical physics, we are striving to address various facets of these intricate biological processes. Our research includes investigating the mechanisms of protein search on genomic DNA, protecting genomic integrity through ssDNA-protein interactions, decoding genetic information from hierarchical genomic organization, understanding nucleosome biophysics, exploring the role of DNA supercoil topology, and much more. |
|
|
|
||
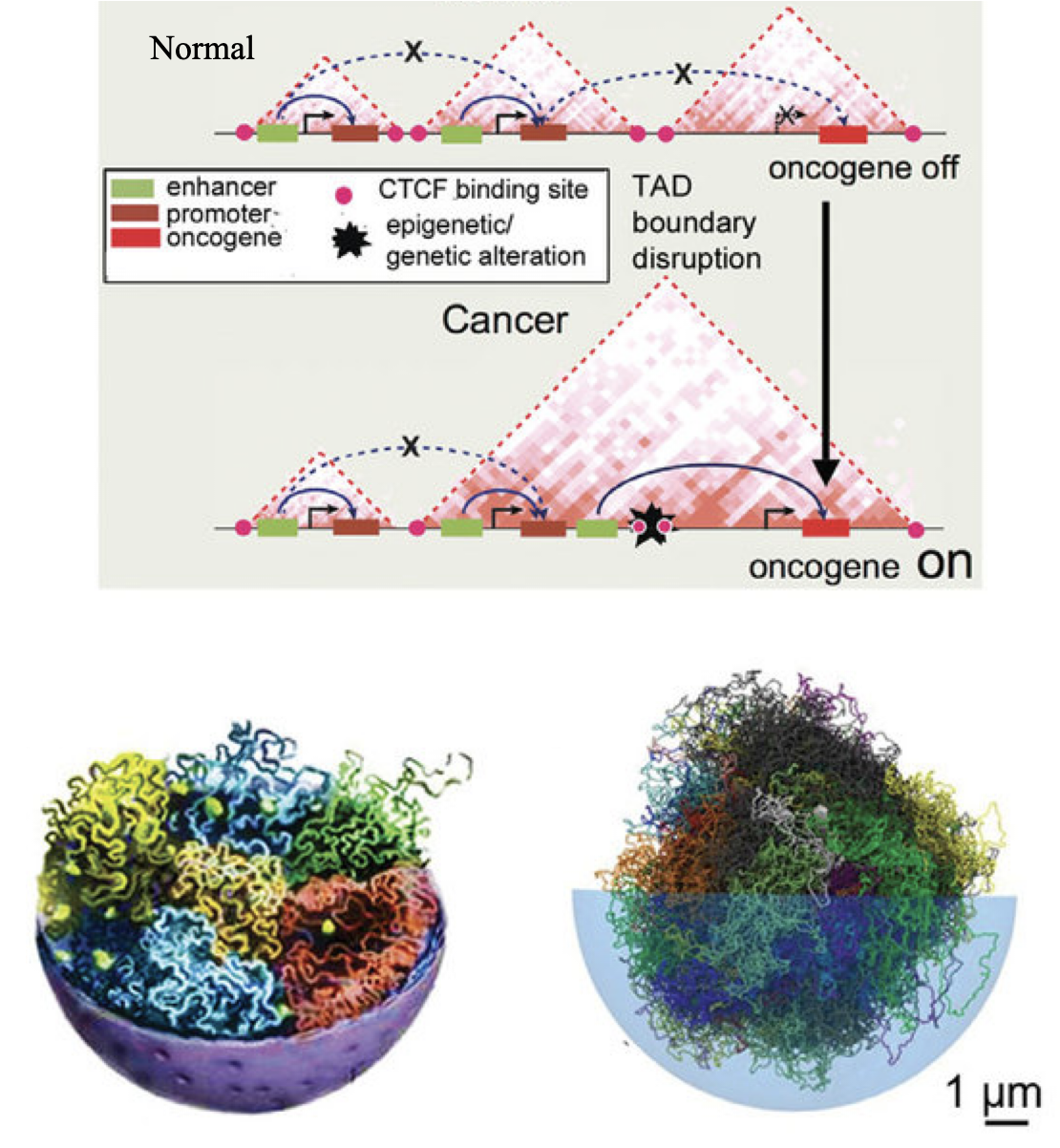 |
Hierarchical Organization of Genome Structure and Its Functional ImplicationsThe genomes of higher eukaryotes are organized into complex structures spanning multiple length scales. At the largest scale, chromosomes can be easily observed under a standard light microscope. At the smallest scale, nucleosomes—comprising DNA wrapped around a core of eight histone proteins—represent the fundamental units of chromatin. These nucleosomes further organize into chromatin fibers, leading to a highly compact genome configuration. This hierarchical packaging tightly regulates the accessibility of regulatory DNA sequences (genes) by DNA-binding proteins (DBPs). The spatial positioning of genes within the nucleus is intricately linked to gene regulation, warranting detailed investigation to unravel the underlying mechanisms. However, this task is challenging due to the inherently multiscale nature of chromatin and our limited understanding of how chromatin organization influences gene recognition by transcription factors. To address these challenges, we employ multiscale polymer simulations alongside advanced machine learning techniques. Our goal is to bridge the current knowledge gaps that hinder our understanding of the molecular mechanisms of gene regulation under nuclear constraints. We aim to decode the complexities of the 3D chromatin landscape associated with transcription, aspects of which have not been adequately captured—or in some cases, not captured at all. Our research seeks to provide novel insights into the structure-function relationships within the genome, with significant implications for fundamental biology and medical science, particularly in understanding developmental disorders and cancers arising from gene misexpression. |
|
|
|
||
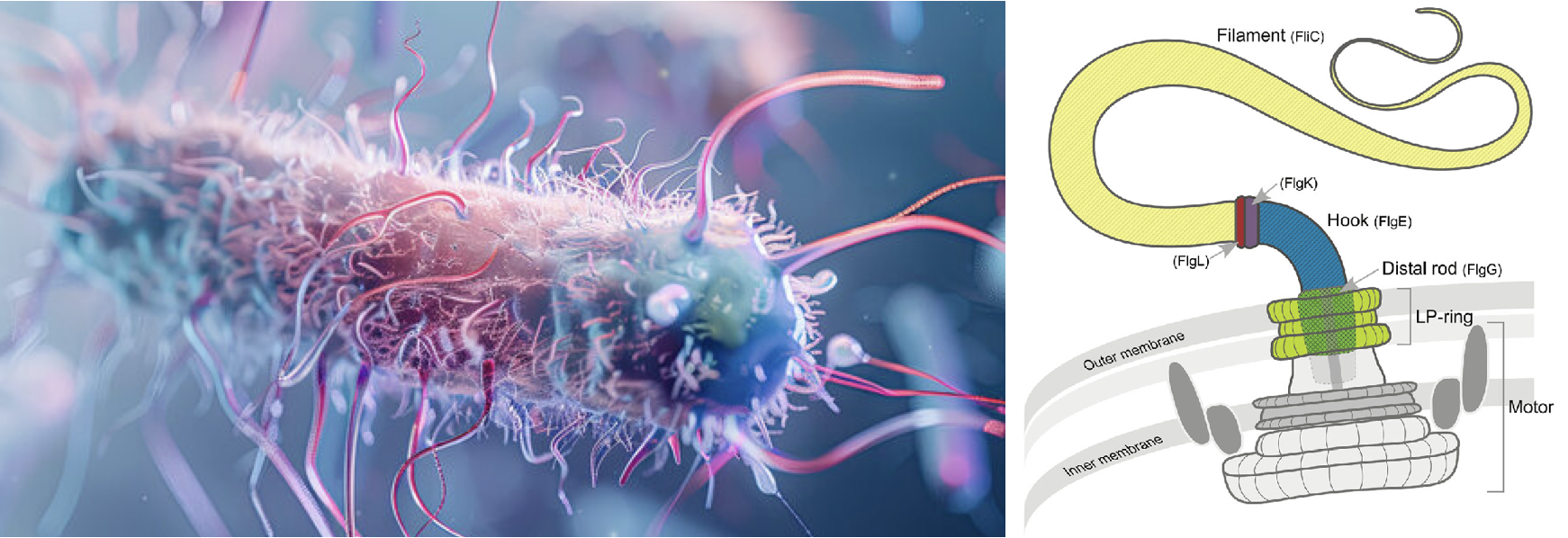 |
Dynamics and Emergent Behaviour of Active MatterWith Dr. Annwesha Dutta, INSPIRE Faculty at SCIS, we have recently embarked on a research project focused on investigating the Dynamics and Emergent Behaviour of Active Matter, which seeks to unravel the intricate collective behaviours that arise in systems composed of self-driven particles or entities. Unlike passive materials, active matter systems are made up of components that consume energy to move and interact, resulting in rich and often unpredictable dynamics. Examples of active matter include biological systems such as flocks of birds, schools of fish, bacterial colonies, and cellular processes, as well as synthetic systems like active colloids and robotic swarms. Our research employs coarse-grained molecular simulations and principles of statistical physics to delve into the complex behaviors of active flagella, commonly found in viruses. Our primary objectives are to understand the rules that govern these interactions, to explore how order and coherence emerge from disorder, and to investigate how these systems can be controlled or harnessed for technological applications. |
|
